
0.1 M).Įxample #6: In a geographical area at sea level measuring 56 km by 24 km by 1 km, the ozone concentration is 0.145 ppm. You can cut the dilution process to one step if a lower concentration of HCl is available (e.g. HCl / 100 mL) and then dilute THAT solution by 1/225.
#How to calculate ppm by mass serial
The best way to do that is a serial dilution: first make a 1/100 dilution (1 mL conc. If you want 20 ppm, you need to make a 20/449,000 volume dilution, or 1/22,500. We need 1.10 mL of the stock solution diluted to 1.00 L to make a 20.0 ppm solution of HCl.ģ) A slightly different form of the above question was answered thusly on an "answers" website:Ĭoncentrated HCl is 12.3 moles/L or, since the molar mass of HCl is 36.5 g/L, 449 g HCl / L.Ĥ49 g/L = 449,000 mg/L.

We need 20.0 mg solute per liter of solutionĢ) Calculate the volume of 0.500 M solution that contains 20.0 mg HCl:
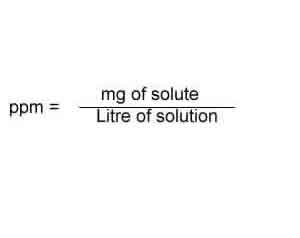
Since ppm = 1 mg solute per liter of solution: Be aware!Įxample #5: Make a 20.0 ppm solution of HCl from a stock solution that is 0.500-molar.ġ) Let us assume we will make 1.00 L of solution. This focus on an ion (and not the entire formula of the substance) in ppm concentrations is common. The weight of the carbonate, based on the wording of the question, is immaterial to the solving of the problem. Notice how the weight for calcium ion only is used. This is the common practice in ppm calculations.Ģ) Convert to ppm by multiplying numerator and denominator by 1000:Ġ.4008 g / 1000 g of solution times 1000/1000 = 400 g / 1,000,000 g of solution Note that the solution density is assumed to be 1.00 g/mL. Moles in one liter = mass/molar mass = 0.00289/522 = 5.5 x 10 -6 molĮxample #4: What is the ppm concentration of calcium ion in 0.010 M CaCO 3?ġ) Convert mol/L to to g/1000 g of solution:Ġ.010 mol/L times 40.08 g/mol = 0.4008 g/LĠ.4008 g/L = 0.4008 g / 1000 g of solution If you work out the mass per liter it makes working out the next steps a little easier, because the final units for molarity will be mol/L.ģ) Now using the molar mass to work out the molarity (moles per litre): Therefore 1 liter (L) of water is 1,000 g. If the solvent is water, we can assume the density at standard temperature and pressure is 1.0 g/mL. What is the molarity of the solution?ġ) Unless specified otherwise, ppm usually refers to ppm by weight:Ģ.89 ppm = 2.89 g per 1,000,000 g (or any other weight unit, I have just chosen g for convenience)Ģ) Now you need to know the density of the solvent to convert the volume to mass. You might want to go back to problem #1 and try out 78 mg/L with the atomic weight of calcium ion expressed as mg/mol instead of g/mol.Įxample #3: A solution is labeled 2.89 ppm and is made with a solute that has molar mass equal to 522 g/mol.


The best way to explain this is by doing some examples.Įxample #1: Convert 78.0 ppm of Ca 2+ ions to mol/Lġ) By the last definition of ppm just above:ħ8.0 ppm = 78 mg Ca 2+ / L of solution = 0.0780 g/LĢ) Divide by the atomic weight for calcium ion:Ġ.0780 g/L / 40.08 g/mol = 0.00195 mol/L (to three sig figs)Įxample #2: Calculate the molarity of a dye concentration given the molar mass is of the dye 327 g/mol and a dye concentration of 2 ppm.ġ) Convert ppm to a gram-based concentration:Ģa) Using 0.002 g/L, caculate the molarity:Ġ.002 g/L divided by 327 g/mol = 6.1 x 10 -6 MĢ mg/L divided by 327,000 mg/mol = 6.1 x 10 -6 M This last one works because the solution concentration is so low that we can assume the solution density to be 1.00 g/mL.Īlso, it's this last modification of ppm (the mg/L one) that allows us to go to molarity (which has units of mol/L). Now, divide both values by 1000 to get a new definition for ppm: ChemTeam: Converting between "ppm" and molarity Converting between "ppm" and molarity


 0 kommentar(er)
0 kommentar(er)
